Methods of synthesis of nanoparticles (NPS) of silver to synthesis graphene oxide (OG)
Авторы: Бурцева Евдокия Константиновна, Кириллина Елена Валерьевна
Рубрика: 2. Электроника, радиотехника и связь
Опубликовано в
V международная научная конференция «Современные тенденции технических наук» (Казань, май 2017)
Дата публикации: 03.05.2017
Статья просмотрена: 56 раз
Библиографическое описание:
Бурцева, Е. К. Methods of synthesis of nanoparticles (NPS) of silver to synthesis graphene oxide (OG) / Е. К. Бурцева, Е. В. Кириллина. — Текст : непосредственный // Современные тенденции технических наук : материалы V Междунар. науч. конф. (г. Казань, май 2017 г.). — Казань : Бук, 2017. — С. 14-17. — URL: https://moluch.ru/conf/tech/archive/230/12349/ (дата обращения: 03.05.2024).
In this article, we demonstrate a simple method for obtaining silver nanoparticles for synthesis with graphene oxide. This method has a number of positive aspects. One of the main ones is not the difficulty of obtaining a suspension of silver nanoparticles. In the reaction with graphene oxide, very good results are obtained in electrophysical properties.
Keywords: graphene, graphene oxide, silver nanoparticles
Graphene with its unique electrical, mechanical, optical and thermal properties, attracts the interest of many researchers. OG is a dielectric and for use in electronics is of little use. To increase the electrical conductivity, it is necessary to carry out a recovery process.
Producing new composite materials based on nanoparticles (NPS) has increased relevance recently due to the fact that the unique properties of woofers, placed in various matrices, persist and even enhance. The most promising application areas of graphene oxide (OG) based materials and noble metals nanoparticles are catalysis, fuel cells, chemical sensors and biological applications.
For the synthesis of gold nanoparticles in this work was used the citrate method. A distinctive feature of this method is that the citrate-anion simultaneously acts as a stabilizer and reductant. This method of obtaining gold nanoparticles is applicable to the preparation of nanoparticles of silver. But since silver is more active metal than gold, the synthesis of silver nanoparticles is more difficult because of the silver’s ability to rapid oxidation and aggregation [1, p. 14177].
The OG suspensions used in this work were obtained by a modified Hammers method [2, p. 87–94]. To prepare a suspension of Au NPS, aqueous solutions of sodium citrate (1 ml) and silver nitrate (0.25 ml) were successively added to 1.25 ml of water with constant stirring at room temperature. After 5 minutes of incubation and addition of boiling water, the color of the solution changed from colorless to cloudy brown. The resulting solution was additionally boiled for 1 hour with continuous stirring in order to maximize the yield of nanoparticles [3, p. 541]. Further, the Au NPS and OG solutions were mixed in a volume ratio of 1: 1. The prepared suspension was applied on a thin layer on the SiO2 surface and a glass plate (for optical measurements) and dried at room temperature for 24 hours. Dielectric substrates were pre-washed with acetone and alcohol in an ultrasonic bath, and to impart hydrophilic properties they were processed in an aqueous solution of hydrogen peroxide and ammonia. The formed membranes had thicknesses from several units to tens of nanometers. For the purposes of the exhaust gas restoration in order to remove the oxygen groups, a thermal treatment at 250o C for 30 minutes in an argon atmosphere and chemical reduction with ascorbic acid were carried out.
|
a) GO_AG-1/3 |
b) GO_AG-1/2 |
d) GO_AG-1/1 |
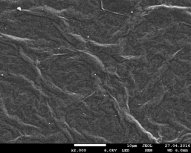
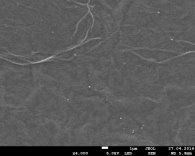
Fig. 1 Image of SEM of Au nanoparticles in OG at ratios of 1/3, 1/2, 1/1 solutions, respectively.
Figure 1 shows the images of the surface of exhaust gas from Au NPS obtained by scanning electron microscope (SEM) JEOL JSM 7800F. It can be seen from the figures that the concentration of Au NPS in the exhaust gas at a ratio of 1/1 is greater than in the cases of 1/2 and 1/3. The smallest concentration is observed in GO_AG-1/3 (fig. 1 a). Au NPS dimensions range from several to several hundred nanometers.
The electron microscopy study showed that on the surface of the ULF they are collected in agglomerates with dimensions of several hundred nanometers (Fig. 2a). X-ray energy-dispersive microanalysis shows a high content of silver atoms in these agglomerates (Table 1). Unlike SiO2, SNF in the volume of exhaust gas is not collected in agglomerates and distributed in the form of separate particles, leading to the formation of wrinkles and wrinkles on the surface (Fig. 2b). As can be seen from Fig. 2, the formed ULFs have an irregular shape, the sizes of which lie mainly in the range from 60 to 200 nm.
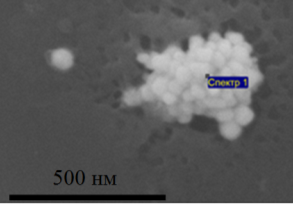
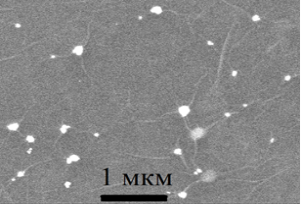
Fig. 2. Agglomerate of silver nanoparticles on the surface of SiO2 (a) and silver nanoparticles in the matrix of graphene oxide (b)
Table 1
|
Element |
Atomic% |
|
С |
50.99 |
|
О |
27.36 |
|
Ag |
21.65 |
Metal nanoparticle-decorated graphene oxides are promising materials in various optoelectronic applications because of their unique plasmonic properties. M-K. Chuang et. al. explained it in their article, as a simple, environmentally friendly method for the synthesis of gold nanoparticle decorated graphene oxide that can be used to improve the efficiency of organic photovoltaic devices (OPVs) is reported [4, p. 19095].
The Suzuki-Miyara method of studying gold nanoparticles made a big breakthrough in modern electronics. This method was used to fabricate gold nanoparticle/ graphene oxide nanocomposites that exhibited unexpected catalytic activity when coupling reaction of chlorobenzene and phenylboronic acid.
Suzuki-Miyaura coupling reactions are often used to test the catalytic performance of Pd or Au NPS in organic chemistry. Chlorobenzene with phenylboronic acid in an aqueous medium have high catalytic activity with Au NPS/GO. The author prepared the Au NPS/GO, for the first time, without any additional reductant. This turned out to have a high catalytic activity for the Suzuki-Miyaura coupling reaction of chlorobenzene with phenylboronic acid in an aqueous medium [5, p. 4455–4458]. N.Zhang, H. Qui, et. al. proved that Au NPS in organic catalytic reaction will have great potential in use.
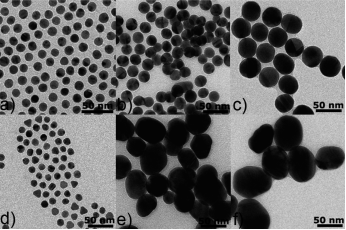
Fig. 3. TEM images of Au NPs obtained by the present method (a=c) and the Turkevich method (d=f) at different citrate concentrations: 2.97x10–2 wt %(a, d), 1.01x10–2 wt %(b, e), and 6.10x10–3 wt %(c, f)
This fig. 3 demonstrates a simple and reproducible way to produce quasi-spherical Au nanoparticles (NPs) with a fairly narrow size distribution in water by rapidly adding a mixture solution of HAuCl4, sodium citrate, and a trace amount of silver nitrate into boiling water. The sizes of quasi-spherical Au NPs obtained increases from 12 nm to 18 and 36 nm with decrease of the citrate concentration in a fairly linear way. In the case of synthesis of Au NPs of sizes ranging from 10 to 36 nm, our approach efficiently makes up the shortages of the classical Turkevich method with respect to the reproducibility and uniformity of the NP size and shape [6, p. 232–240].
Thus, to obtain a solution of silver NPS is not more complicated. The possibilities of studying graphene oxide with silver nanoparticles are unlimited. The introduction of silver NPS leads to an improvement in the multitude of electrophysical properties of OG. Such a result will help to breakthrough nanotechnology.
References:
- Y. Yoon, K. Samanta, H. Lee, K. Lee, A. P. Tiwari, J. Lee, J. Yang, H. Lee., Highly Stretchable and Conductive Silver Nanoparticle Embedded Graphene Flake Electrode Prepared by Insitu Dual Reduction Reaction, Scientific Reports, 2015, vol.5, P. 14177.
- Neustroev E. P., Alexandrov G. N., Nogovitcyna M. V., Influence of Low Temperature Thermal Reduction on Physical and Chemical Properties of Graphene Oxide, Vestnik SVFU, 2015, 6(50), P. 87–94. (In Russ.)
- S. W. Chook, C. H. Chia, S. Zakaria, M. K. Ayob, K. L. Chee, N. M. Huang, H. M. Neoh, H. N. Lim, R. Jamal, R. M. F. R. A. Rahman., Antibacterial performance of Ag nanoparticles and AgGO nanocomposites prepared via rapid microwave-assisted synthesis method, Nanoscale Research Letters, 2012, No. 7, P. 541.
- L. Li, Y. Guo, X. Zhang and Y. Song // Mater. Chem. A. — 2014. — No.2. — P. 19095.
- P. P. Zhang, X. X. Zhang, H. X. Sun, R. H. Liu, B. Wang and Y. H. Lin, Tetrahedron Lett., 2009, 50, P. 4455–4458.
- W. Zhang, E. Bi, M. Li, L. Gao. Synthesis of Ag/RGO composite as effective conductive ink filler for flexible inkjet printing electronics, Colloids and Surfaces A: Physicochem. Eng. Aspects, 2016, No. 490, P. 232–240.
Ключевые слова
графен, Оксид графена, Наночастицы серебра, graphene, graphene oxide, silver nanoparticlesПохожие статьи
Indium Nanoparticles by pulsed plasma | Статья в журнале...
The electron diffraction patterns of the products were taken by Transmission Electron Microscopy (TEM) JEOL-200FX (Kumamoto University, Japan) and JEOL JEM-1400 (CNBM, Adam Mickiewicz University, Poland)...
Фотокаталитические свойства наноразмерного оксида цинка...
The morphology of the nanostructures was studied by scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
электронном микроскопе (ПЭМ) JEOL JSM-6490LA (университет Кумомото, Япония) и растровом электронном микроскопе JSM-5310LV (СЭМ).
Моделирование смешанной адено-герпетической инфекции клеток...
Ультратонкие срезы, полученные на ультрамикротоме LKB 8800, контрастировали уранилацетатом и цитратом свинца и исследовали на микроскопе JEM-1400 (Jeol, Япония).
Electron microscopy: methods and protocols / Edited by John Kuo.–
Методы формирования пористого кремния с различным размером...
Растровые изображения сколов образцов были получены на электронном микроскопе компании JEOL — JSM 6380LV (Рис. 1 и 2). На Рис. 1 представлены изображения пористого кремния полученного на подложках марки КЭФ <100> (a — образец 3) и
Journal of Non-Crystalline Solids.
Похожие статьи
Indium Nanoparticles by pulsed plasma | Статья в журнале...
The electron diffraction patterns of the products were taken by Transmission Electron Microscopy (TEM) JEOL-200FX (Kumamoto University, Japan) and JEOL JEM-1400 (CNBM, Adam Mickiewicz University, Poland)...
Фотокаталитические свойства наноразмерного оксида цинка...
The morphology of the nanostructures was studied by scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
электронном микроскопе (ПЭМ) JEOL JSM-6490LA (университет Кумомото, Япония) и растровом электронном микроскопе JSM-5310LV (СЭМ).
Моделирование смешанной адено-герпетической инфекции клеток...
Ультратонкие срезы, полученные на ультрамикротоме LKB 8800, контрастировали уранилацетатом и цитратом свинца и исследовали на микроскопе JEM-1400 (Jeol, Япония).
Electron microscopy: methods and protocols / Edited by John Kuo.–
Методы формирования пористого кремния с различным размером...
Растровые изображения сколов образцов были получены на электронном микроскопе компании JEOL — JSM 6380LV (Рис. 1 и 2). На Рис. 1 представлены изображения пористого кремния полученного на подложках марки КЭФ <100> (a — образец 3) и
Journal of Non-Crystalline Solids.