В данной статье рассматривается некоторые особенности применения реакции Фишера — Тропша, а также оборудование того времени для получения синтетического топлива.
Ключевые слова: война, реакция Фишера — Тропша, топливо, Германия
Introduction
War devours resources, and the most coveted resource, the basis of the war economy, is petroleum (petrol, diesel, jet fuel). Although science helped Hitler and the Reich got 20 million litres of fuel a year from low-grade lignite with the help of Fischer — Tropsch technology, the Reich still lacked petrol, diesel and jet fuel for its army of millions. This was the reason for the Battle of Stalingrad. The Reich needed the oil of the Caucasus, but in order to get it, the Wehrmacht leadership could not afford to be surrounded, which would inevitably have happened if it had left Stalingrad behind.
Let us now analyse in detail how they got this petrol and take a closer look at the first-generation refineries, now improved to fourth generation, successfully producing hundreds of millions of litres of petrol, diesel and other hydrocarbons around the world.
A brief theory
Everyone knows how petrol is made from crude oil — namely, it is «cooked» in closed tanks. The same way dirty oil can be made today is by boiling it in closed barrels (called retorts) over a fire from old tyres. Or from ordinary household rubbish. Yes, that's right, if you put rubbish (any rubbish from the bin) in a closed barrel (hermetically seal it) and light a fire under it, you can get liquid dirty oil. Of course, this «oil» is dirty, and to distil it into clean fuel will require a lot of equipment, cleaning, and in the end the output of clean fuel will be very low, and a lot of energy will be used, both electrical and thermal (gas to keep the process going). The equipment will be cumbersome and expensive. This is why there are wars going on in the world for quality oil from which petrol and diesel can be distilled at the lowest possible cost. Frans Fischer and Hans Tropsch, who worked on the problem of petrol in the 1920s, took a different approach — they wanted to obtain petrol and diesel not by «cooking» the raw materials, but by catalysis [1]. How does this work?
Petrol is made from a solid fuel, which can be anything that can burn.
The solid fuel is first gasified in gas generators, converting it into syngas, and then the syngas is passed through a milled catalyst made of a certain iron, and the gas is converted into petrol, making diesel very clean — cleaner than at the petrol station.
The essence of gasification is that 20 per cent of the fuel is simply burnt and at the same time 80 per cent of the fuel is converted into synthesis gas by the heat of combustion of the fuel. In a nutshell, this process works like this: we take the smoke from the fire (burning fuel in a gas generator is the same as burning it in a conventional fire) and pass it back through the hot coals, and when this is done in a closed container, without drawing in air from outside, the smoke becomes combustible gas. It is as simple as that (it is true that it is difficult to calculate the apparatus according to formulae and then purify the gas).
Since there were and are large lignite deposits in Germany, and since extraction was done industrially then (as it is now), it is easier to make petrol from lignite. If there were industrial extraction of unlimited quantities of wood, more would be extracted from wood than from coal. Incidentally, it would be easier and cheaper to extract synthesis gas from wood because wood does not contain sulphur, whereas coal does, and cleaning sulphur from coal is an additional industrial operation to clean organic sulphur and hydrogen sulphide. Sulphur and oxygen kill the Fischer — Tropsch catalyst because they are catalytic poisons [1] [2].
It is much more interesting to convert combustible fuels that have a low price or waste that costs money to remove and dispose of, such as rubbish or faeces from the municipal sewage system, into petrol and diesel than to extract them from petroleum — which, as Mendeleev famously said, is like «burning money».
Scientists have spent decades searching their laboratories for metals suitable for catalytic reactions. Only four metals suitable for converting syngas into gasoline were found, two of which were discarded, and two are still used — iron and cobalt (nickel and ruthenium were discarded).
The scientists racked their brains and this is what the first apparatus for producing liquid hydrocarbons looked like.
In Fig. 1 you can see the «heart» of the machine, where converted coal is turned into synthesis gas and synthesis gas is turned into petrol and diesel (we will look at the whole machine in more detail). There are 1.5 mm thick sheets lined up on the pipes and the distance between the pipes is small. Why is this done and why exactly?
The fact is that when syngas is converted into petrol (when blown through catalyst powder or balls with powder applied), energy is released that is equivalent to 30 % of the energy obtained when the gas is ignited. Each gas cube thus yielded the equivalent of about 600 kcal/Nm3. If this process were allowed to proceed without cooling, the temperature would reach almost 1500 degrees Celsius. And the temperature had to be about 210 degrees Celsius for the process to run properly. For this reason, steam was fed into the pipes to dissipate the excess heat. Fischer's own experience was that the distance between the plates in such a setup should not exceed 10 mm, and he managed 7 mm. For every litre of petrol obtained (I refer here to all liquid hydrocarbons obtained in this way as petrol and further also), 5 kg of steam should be added. The pipes had a slightly smaller diameter than the water pipes in our homes and a wall thickness of 4 mm so that they would not break under the pressure, which could reach up to 30 atmospheres.
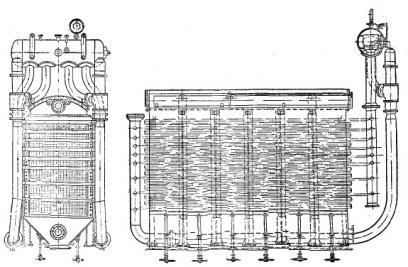
Fig. 1 Schematic representation of the Fischer — Tropsch plate reactor at atmospheric pressure
One such reactor produced 1.9t of petrol per day and about 6000kg of steam.
One cube of synthesis gas produced 160g of gasoline. Considering that 1kg of lignite gave about 3.7m3 of gas we can calculate the economics.
Reactor was 5 metres long and up to 3.5 metres high. Reactor width was 2m. About 3 tons of catalytic agent — made on iron base — was being loaded into reactor at a time. The weight of such unit was up to 50 tons [1] [2].
References:
- Karakhanov E. A. Synthesis gas as an alternative to oil. 1. Fischer — Tropsch process and oxo-synthesis // Sorosov's Educational Journal. 1997. № 6
- Kummer J. T. Some Mechanism Studies on the Fischer — Tropsch Synthesis Using C14 / J. T. Kummer, T. W. DeWitt, P. H. Emmett // Journal of American Chemical Society. — 1948. — Vol. 70. — № 11. — P. 3632–3643.







