Introduction
Quantum dots (QDs) or semiconductor nanocrystals are a type of semiconductor nanoparticles with sizes ranging from 2 to 10 nm. QDs have unique fluorescent properties such as good fluorescence stability, broad spectrum light absorption, and narrow emission spectrum. Therefore, QDs have potential applications in many fields such as electronic engineering, solar cells, biological imaging techniques... However, they are often made from materials such as cadmium selenide (CdSe) [1] [2], [3] cadmium sulfide (CdS) [4], zinc sulfide (ZnS) [5], zinc selenide (ZnSe) [6]. This raises concerns about toxicity and high cost. Finding materials with optical properties similar to QDs but environmentally friendly is a matter of special interest and priority for scientists.
Conventional carbon is a black material that is poorly soluble in water and has no fluorescence. Nanoscience creates great opportunities to expand science and technology, such as synthesized carbon nanostructures that have properties completely different from macro-materials. Carbon nanodots (CDs) are a new generation of nanomaterials. CDs have broad emission spectra, controllable fluorescence, excitation wavelength-dependent emission, and up-conversion [7]. Compared with commercial quantum dots, CDs have the outstanding advantages of low toxicity and easy fabrication. They are promising as fluorescent labeling agents in cell imaging, bioimaging, and also find applications in photocatalysis, energy storage, optoelectronics, and drug delivery [7].
Raw materials for making CDs include artificial carbon sources such as graphite [8], candle soot [9], ammonium citrate [10],… Recently, scientists have focused on researching and manufacturing carbon nanodots from natural sources. Natural materials have advantages such as being abundant, low cost, renewable and having good biocompatibility. In fact, CDs have been successfully made from orange juice [11], coffee grounds [12], soy milk [13], carrot juice [14],...
CDs can be manufactured by different methods such as electrochemistry, chemical oxidation, hydrothermal... Hydrothermal method is considered a method of manufacturing materials with low cost, environmentally friendly, and easy to control the size of the material.
This paper presents a method for preparing CDs from passion fruit juice. The obtained material is well dispersed in alcohol. In particular, in this solvent, the fluorescence intensity of CDs increases significantly compared to the pure material.
Materials and methods
Passion fruit was purchased from a supermarket. The passion fruit juice after removing the seeds was diluted with distilled water in a 1:1 ratio. The diluted passion fruit juice was poured into a thermowell, placed in an oven and heated at 200 o C for 12 h. After being removed from the oven, the thermowell was allowed to cool naturally. The product was filtered through a 2-micrometer filter membrane to remove any mixed carbon.
The material properties were analyzed by infrared spectroscopy (FTIR), X-ray diffraction (XRD). The fluorescence spectrum of the sample was recorded by a Nano Log fluorescence spectrometer (Horiba, Edison, USA).
Results and discussions
The main components of passion fruit juice are carbohydrates (23 %) and water (75 %). Carbohydrates in lemons usually include fiber and simple sugars sucrose, fructose, glucose. In addition, passion fruit juice also contains ascorbic acid. The formation of carbon nanodots from passion fruit juice can be described as follows: (i) carbohydrates are hydrolyzed, dehydrated and decomposed in the presence of ascorbic acid to form soluble compounds as furfural compounds (for ex: 5- hydroxymethyl furfural, furfural, 5-methyl furfural,…), several organic acids such as acetic, lactic, propionic, livulinic and formic acids, aldehydes and phenols (ii) in the next stage, polymerization and condensation of the above compounds take place to form soluble polymers. Aromatization and formation of aromatic clusters take place through aldol condensation, ring addition, and indirect hydroxymethyl condensation via furan. (iii) When the concentration of aromatic clusters reaches the standard supersaturation point, carbon nucleation occurs [11]. The growth of carbon clusters is assisted by the diffusion of other molecules towards the particle surface. The result is the formation of C-dots which are self-passivated by the H and O containing groups.
Figure 1 shows the HRTEM image of CDs fabricated at 200 o C for 12 h. Due to the low contrast between the CDs and the carbon-coated copper grid, the images of the CDs samples are difficult to distinguish. However, the distinct black spots observed in the image demonstrate the formation of isolated CDs. These clusters appear to be spherical in shape with sizes ranging from 15 to 20 nm.
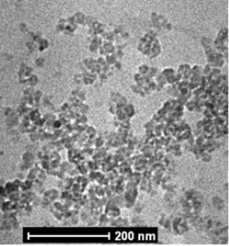
Fig. 1. High-resolution transmission electron micrograph of CDs
The degree of crystallinity of the material is shown through the X-ray diffraction pattern in Figure 2. In this pattern, only a low broad diffraction peak at about 22 o is observed, indicating the disordered arrangement in the core of CDs [11]. Although many scientists have demonstrated the existence of crystalline sp 2 carbon regions, most of CDs extracted from natural products have poor crystallinity.
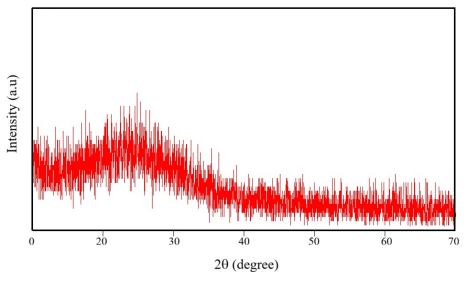
Fig. 2. X-ray diffraction pattern of carbon nanodots
Passion fruit juice after hydrothermal treatment gives a clear dark brown solution. When the solution is irradiated with ultraviolet rays, CDs emit green light (Figure 3).
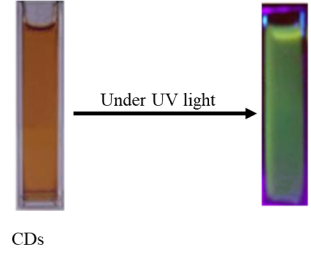
Fig. 3. Images of carbon nano dots under normal light and ultraviolet light
The fluorescence spectrum of CDs has a broad emission band extending from violet to yellow (Figure 4). The maximum emission wavelength is in the range of 530–540 nm. In addition, the emission spectrum is asymmetric with a small tail extending into the longer wavelength region. These phenomena suggest that the fluorescence properties of CDs are related to diverse surface states [15].
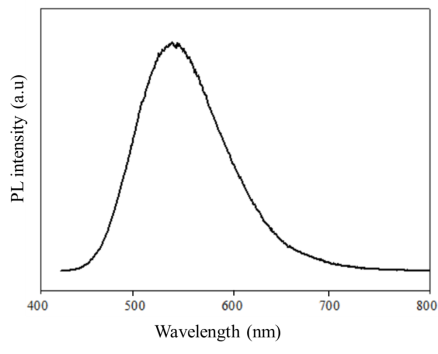
Fig. 4. Fluorescence spectrum of carbon dots
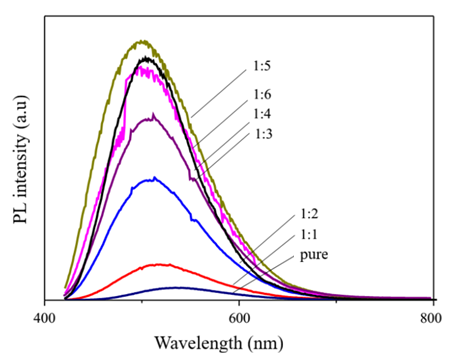
Fig. 5. Fluorescence spectra of CDs diluted with alcohol
The fluorescence properties of CDs have also been studied in alcohol. Figure 5 shows the emission spectrum of CDs when diluted with alcohol at varying volume ratios from 1:1 to 1:6. It was observed that the emission intensity of CDs in alcohol increased significantly compared to pure CDs. As the volume ratio of CDs to alcohol decreased from 1:1; 1:2; 1:3; 1:4; 1:5 (CDs concentration in water decreased), the emission intensity gradually increased. The greater the amount of alcohol added, the lower the probability of collisions between CDs dots. Therefore, the energy loss of CDs due to collisions decreased. In addition, diluting CDs also reduced self-absorption in high-concentration solutions. As a result, the lower the concentration of CDs in the solution, the higher the emission intensity. However, when the concentration of CDs is too low (ratio of 1:6 or higher), the number of emission centers in the solution is reduced, and therefore the emission intensity decreases. This result was also observed in CDs fabricated from onion peels [16].
Conclusion
Carbon nanodots were fabricated from passion fruit juice using a hydrothermal method. The CDs readily disperse in alcohol and emit green light. The emission intensity of the CDs was highest when diluted with alcohol at a volume ratio of 1:5.
References:
- Karan Surana, Pramod K. Singh, Hee-Woo Rhee, Bhaskar Bhattacharya. Synthesis, characterization and application of CdSe quantum dots. Journal of Industrial and Engineering Chemistry, 2014, Vol. 20, No. 6, pp. 4188–4193
- А. K. Jigyasu, S. Siddiqui, M. Lohani, I. A. Khan, and M. Arshad (2016). Chemically synthesized CDSE quantum dots inhibit growth of human lung carcinoma cells via ros generation. EXCLI J, Vol. 15, pp. 54–63
- Anna Kiczor and Paweł Mergo (2024). Synthesis of CdSe Quantum Dots in Two Solvents of Different Boiling Points for Polymer Optical Fiber Technology. Materials, Vol. 17, No. 227
- Srivani Veeranarayanan et al (2012). Synthesis and application of luminescent single CdS quantum dot encapsulated silica nanoparticles directed for precision optical bioimaging. International Journal of Nanomedicine, Vol. 7, pp. 3769–3786
- Weiquan Yuan et al (2022). Facile synthesis and characterization of ZnS polymorphs/Halloysite composite for efficiently selective adsorption of Al(III) from acidic rare earth ions solution. Sep Purif Technol , Vol. 291, p. 120849,
- S. H. Duy Hoang Nguyen et al (2024). Reaction-dependent optical behavior and theoretical perspectives of colloidal ZnSe quantum dots. Sci Rep, Vol. 14, p. 13982
- D. P. Mychele Jorns (2021). A Review of Fluorescent Carbon Dots, Their Synthesis, Physical and Chemical Characteristics, and Applications. Nanomaterials (Basel), Vol. 11, No. 6, p. 1448
- Qinlong Wang et al (2012). Microwave–hydrothermal synthesis of fluorescent carbon dots from graphite oxide. Carbon N Y, Vol. 49, No. 9, pp. 3134–3140
- B. K. Ganesan et al (2024). Candle soot derived carbon dots as potential corrosion inhibitor for stainless steel in HCl medium. J Appl Electrochem, Vol. 54, pp. 89–102
- . W. Jiayue Geng et al (2022). Intrinsic specificity of plain ammonium citrate carbon dots for Helicobacter pylori: Interfacial mechanism, diagnostic translation and general revelation. Mater Today Bio, Vol. 15, p. 100282
- B. B. Swagatika Sahu et al (2012). Simple one-step synthesis of highly luminescent carbon dots from orange juice: application as excellent bio-imaging agents. Chem Commun, Vol. 48, pp. 8835–8837
- Z.-Y. Pin-Che Hsu et al (2012). Synthesis and analytical applications of photoluminescent carbon nanodots. Green Chemistry, vol. 14, p. 917
- J. Z. Chengzhou Zhu et al (2012). Bifunctional fluorescent carbon nanodots: Green synthesis via soy milk and application as metal-free electrocatalysts for oxygen reduction. Chemical Communications, DOI: 10.1039/c2cc33844k
- Y. L. Yang Liu et al (2017). Green synthesis of fluorescent carbon dots from carrot juice for in vitro cellular imaging Original Articles Article Info. Carbon Letters, Vol. 21, pp. 61–67
- Carb Zhang S X et al (2014). Carbon dots with continuously tunable full-color emission and their application in ratiometric pH sensing. Chem. Mater., Vol. 26, pp. 3104–3122
- G. B. Bandi R et al (2016). Facile and green synthesis of fluorescent carbon dots from onion waste and their potential applications as sensor and multicolour imaging agents. RSC. Adv. , Vol. 6, pp. 28633–28639.

