The research provides an electrocoagulation process for the removal of copper from water with the aluminum electrode. The studies were carried out as a function of pH, and current density and the dose of the electrolyte. The results showed that the maximum removal efficiency of 99,4 % Cu with energy consumption of 0,0084 kWh was achieved at pH: 5–6, the inner — electro distance 1cm, current density: 0,01A/cm2 and the dosage of NaCl (electrolyte): 2g/L.
Keywords: electrocoagulation, copper, electrolyte, energy consumption
Electrocoagulation (EC) is an electrochemical method involving in-situ generating of coagulants by electro-dissolution of a soluble anode submerge in the effluent. When a direct current voltage is applied, the anodes of aluminum or iron dissolve to produce Al3+ or Fe2+ ions. The electrochemically generated metallic ions hydrolyze near the anode to form a series of metal hydroxides and/or polyhydroxides that are able to destabilize dispersed particles such as heavy metals such as copper, zinc, lead etc present in the wastewater to be treated [1].
When aluminium is used as anode, the reactions are as follows:
At the cathode,
2H2O+2e−→H2(g)+2OH− (1)
At the anode,
Al→Al3++3e− (2)
In the solution,
Al3+(aq)+3H2O→Al(OH)3+3H+(aq) [2] (3)
The aim of this research was to investigate the possibility of copper removal under different operational variables (pH, current density, inter-electrode distance and the dosage of electrolyte) from the aqueous solution by applying the EC method.
Materials and methods
Chemicals
Cu(SO4)2 (Sigma-Aldrich), NaOH, H2SO4, and NaCl (Xichlong, China)were used to prepare aqueous solutions for the experiments. The pH of the solutions was adjusted by adding 0,1 M HCl or 0,1 M NaOH.
Experimental procedures
Electrocoagulant (EC) experiments were conducted in a lab-scale EC cell having a total volume of 1L. The EC cell was made from standard glass with the size of 10mm (width) × 12mm (length) ×15mm (height) and was equipped with two parallel monopolar electrodes: one anode and one cathode with the dimension of 2mm × 7mm × 17 mm, made of aluminum plate. A digital AC power supply (Itech, IT6952A, 0 ~ 60V, 0~10A, 600W) was used to give alternative current to the EC cell. Before each run, the aluminum electrodes were washed with HCL 0,1M to remove surface matter; then washed again with distilled water, dried and weighted. All runs were performed at room temperature (25–27o C) and with 1L of the aqueous solution during 20 min. At the end of the run, the solution was filtered and then the filtrate was analyzed and the electrodes dried, and reweighted.
Analytical method
The copper was analyzed using UV–Visible Spectrophotometer (Hach, Dr 5000, US) using Hach kit. pH was conducted by Eutech, Singapore.
Removal copper efficiency and electrode consumption were determined using following equations: Cu2+ removal (%) = ![]() (Eq.1)
(Eq.1)
Where: C0: the initial Cu2+ concentration (mg/L) and Ct: the mole of Cu2+ after treatment (mg/L)
Electronical consumption (kWh)W =
Where: A– is the molecular weight of aluminium; n- is the number of electron involved, and F is the faraday constant (96485.3 C mol-1); m — the weight of alumininum dissovle (g)
Results and discussion
Effect of pH
It was true that the initial pH of the solution is one of the important factors that affecting the performance of electrochemical processes in electrocoagulation of the tank. To evaluate its effect, a series of experiments were performed which the distance between two electrodes 1cm, using 100 mg/L copper-containing solutions, with an initial pH varying in the range of 2– 8. From Fig. 1, it can be seen that the removal efficiency of copper was increased with increasing the pH and the maximum removal efficiency of 91,42 % was obtained at pH 7–8. However, copper removal could be increased by the hydrogen generation at the electrocoagulation cathodes and the liberation of hydroxide ions from copper hydroxide at pH≥7. Thus, pH=4–5 was optimal for this electrocoagulation process.
Effect of current density
The current density is an important operating factor which determines the coagulant dosage. The investigated current densities were 10; 30; 50; 80 and 100 mA/cm2. Fig. 2 was determined the effect of current density on the removal efficiencies by the following conditions: pH 4–5, the inter-electrode distance of 1cm and 20 min electrolysis time. As shown in Fig. 2, the removal efficiency of copper at current density 10 to 100 mA/cm2 increase from 76 to 93,7 %. The reason was that when the increase of current density, the extent of anodic dissolution of aluminum increases, resulting in a greater amount of hydroxide flocs for the removal of pollutants. Moreover, the rate of bubble-generation increases and the bubble size decreases with the increasing of current density, resulting in a faster removal of pollutants by H2 flotation.
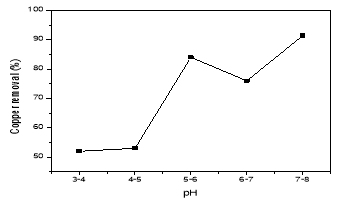
Fig. 1. The effect of pH
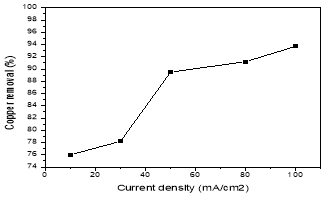
Fig. 2. The effect of current density
Effect of inner- electrode distance
The Fig. 3 was illustrated the effect of distance between two electrodes by the experimental conditions: pH 4–5, current density: 100mA/cm2 in 20 min. The results showed the further the distance, the fewer efficiency of copper treatment. As the distance between electrodes becomes lower, more electrochemically generated gas bubbles bring about turbulent hydrodynamics, thereby leading to a high mass transfer as well as to a high reaction rate between the coagulant species and pollutants. In addition, inter-electrode gap defines the residence time between the anode and the cathode for a continuous system and the time of treatment for a batch reactor for reaching a desirable EC efficiency [3]
The effect of electrolyte (NaCl)
The removal efficiency for copper was 92,2; 92,4; 92,6 % for 1, 2, 3 g/L of NaCl respectively. Because NaCl is a strong electrolyte as below reaction (4). The electrolyte increased the conductivity of the solution, the solubility of the aluminum anode so that the coagulators were generated more and more. At the same time, the power oxidant HClO- was appeared as below reactions (5–6)
NaCl →Na++ Cl- (4)
2Cl- ->Cl2 + 2e- (5)
Cl2(g) +H2O ->HOCl +H++Cl- (6)
To economize of NaCL, mNaCL=2g was chose for the next experiment.
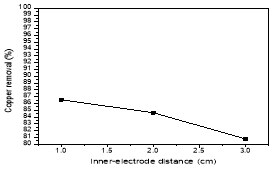
Fig. 3. Effect of inner-electrode distance
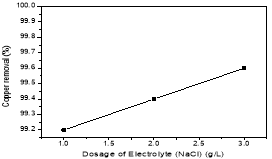
Fig. 4. Effect of the dosage of electrolyte
The consumption of electricity
The efficiency of copper removal was 99,4 % when pH=4–5; inner-electrode distance for 1cm; the current density for 100mA/cm2 and mNaCl 2g/L. The electrical consumption was 0.00894 kWh with 0,25 g aluminum weight for dissolving.
Conclusion
The results show that the maximum removal efficiency of 98.8 % was achieved at a current density of 100 mA/cm2, pH of 4–5, the inter-electrode distance for 1cm and the dosage of NaCl 2g/Lwhen using aluminum alloy as the anode and the cathode. The electrical consumption was 8,4 Wh more economical than other research.
References:
- G. Z. Kyzas and K. A. Matis, “Electroflotation process: A review,” J. Mol. Liq., vol. 220, pp. 657–664, 2016.
- S. Vasudevan, J. Lakshmi, and M. Packiyam, “Electrocoagulation studies on removal of cadmium using magnesium electrode,” J. Appl. Electrochem., vol. 40, no. 11, pp. 2023–2032, 2010.
- J. N. Hakizimana et al., “Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches,” Desalination, vol. 404. pp. 1–21, 2017.







